Do you have kids? Then you know how easily they catch a cough. You’ll likely run to the next pharmacy and grab a bottle of some sweet tasting syrup with a cute cartoon-character on it. A spoonful in the morning and a spoonful at night and they should be o.k.
Mom’s just want their children to be healthy and happy. That’s what the mom’s of 66 children in Gambia, western Africa wanted when they bought cough syrup from an Indian brand for their children in September 2022. These poor kids not only stopped coughing, they eventually stopped breathing altogether, forever. The medicine contained excessive amounts of ingredients that are toxic to humans in these levels.
Counterfeit drugs in the pharmaceutical supply chain
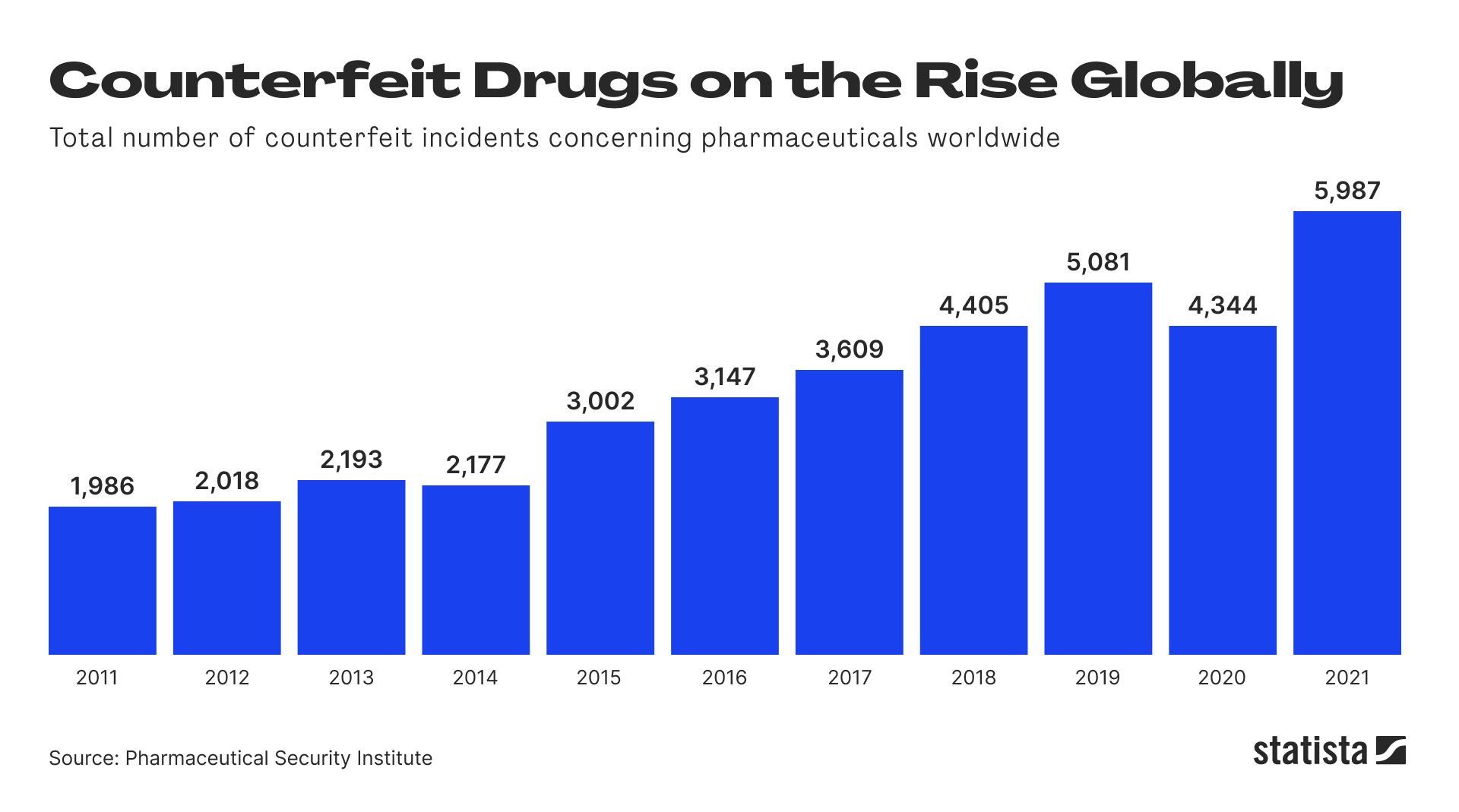
Unfortunately this is not an isolated case of substandard or counterfeit pharmaceuticals leading to the death of unsuspecting patients. According to the World Health Organization WHO, 10.5% of medications in the world are fake or subpar and the numbers are rising.
In the best case these drugs don’t take any effect, in the worst case the consequences can be fatal.
There are other ghastly examples that raise serious questions about the integrity of the pharmaceutical industry. A study from 2004 revealed that 53% of malaria treatment tablets artesunate sold in southeast Asia contained no active ingredient at all. Also, in Cambodia 60 % of malaria treatments were found to be counterfeit. Every year, 155K children die as a result of fake antimalarial drugs.
In Niger, Africa, counterfeit meningitis vaccines lead to 2500 deaths and in Haiti 100 fatal cases of kidney damage were attributed to counterfeit drugs.
Only 50% of drugs are being blocked by anti-counterfeiting measures. The problem affects everybody, including patients, manufacturers, distributors, insurances and they all should have an interest in solving it. Much of it has to do with the lack of traceability and transparency of data in the pharmaceutical supply chain. So what is the problem and how can blockchain improve pharmaceutical supply chain management?
The unique challenges of the pharma supply chain
Every supply chain has two levels: the physical material flow and the information flow that runs parallel to it. Both need to be synchronized. In the pharmaceutical industry, data integrity is critical and a lack of it can cost lives. Keep this in mind when we speak about blockchain in the pharmaceutical industry and in supply chain management later.
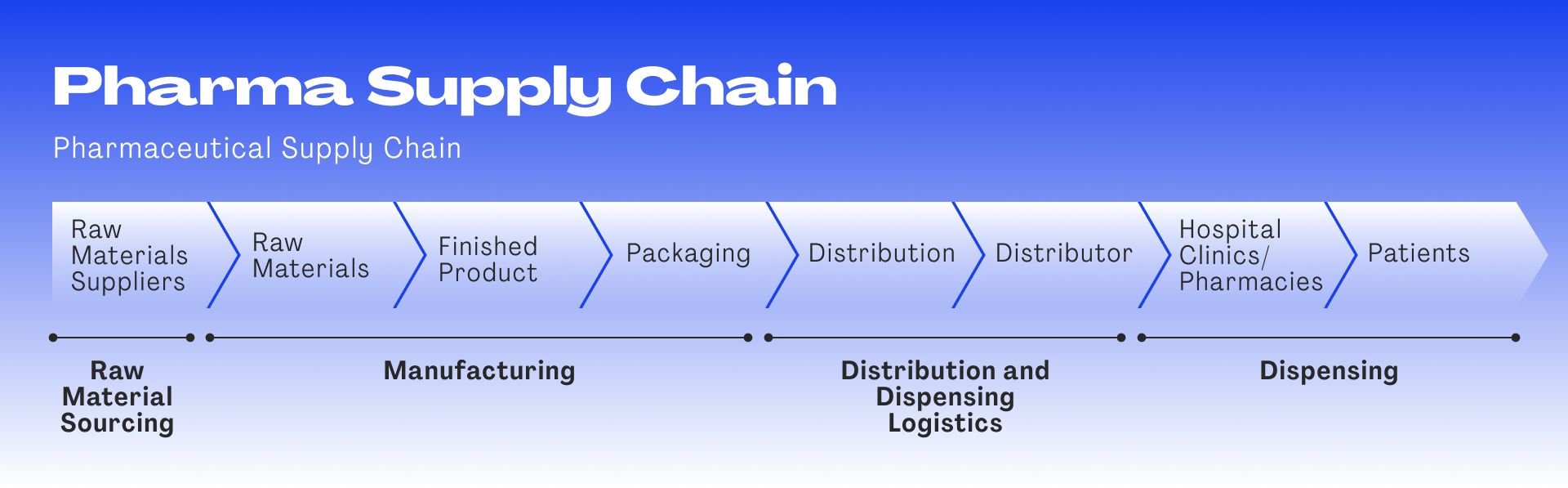
- Many steps and many actors
The pharmaceutical supply chain involves many steps and multiple players at each one. Each intersection where material switches hands, is a potential weak point where information can be forged, changed, erased or added. It’s difficult to identify such data manipulation – whether done on purpose or due to human error – in a system where information is visible only one step up and one step down the chain. Each transaction is a potential breach point.
Blockchain technology enables secure transactions of data and value. When you realize this, you start to understand the potential of blockchain in pharmacy.
- Production losses
To make things more complicated, there are a lot of write offs during the production process. In an industry where the slightest deviation determines the consumer’s health, quality assurance follows extremely strict rules. In case of a recall from the manufacturer, every single package of the medication must be identified and located, the holder informed and made to comply.
Now try to imagine the complexity and amount of transactions involved.
- Storage and transportation
Also packaging and transportation require special care due to the sensitivity of products to temperature, or humidity. 28% of pharmaceuticals are temperature sensitive and conditions need to be monitored during transportation and storage. The pharmaceutical industry loses 35B each year due to temperature deviation during shipping.
Vulnerability lurks also where there’s no transaction, but real-time, accurate data monitoring is required. Blockchain in the supply chain can ensure sensory data cannot be tampered with manually.
- Removing damaged goods
These damaged products need to be removed from the supply chain in an organized, controlled manner, based on an accurate information flow. Removing damaged or unfit pharmaceuticals from the market is almost as important as supplying good ones. Someone might try and resell them, if they are not destroyed.
Tracking every single package of medication and each related transaction in a reliable way is critical for this to work.
- Managing returns
Add to the complexity the returns due to mistakes in orders or shipping. Adequate environmental conditions need to be maintained in return shipments as well, only now the time plays a significant role due to short expiry dates of certain medicines.
Additional data, more intricate transactions, and an increasingly entangled chain.
Material often flows back and forth through the chain, making it more difficult to track with accuracy and leaving the data flow open to a lot of vulnerability. Any delay in data transmission or breach are an opportunity for bad players.
Serialization, track and trace and the blockchain opportunity
The issue is well known to authorities around the globe. The UN has listed quality, reliable, sustainable and resilient infrastructure among its Sustainable Development Goals (SDGs). Supply chains are a significant component of infrastructure and governments have since tightened regulations with focus mainly on serialization and labeling. The idea is to implement electronic systems to track and trace pharmaceuticals.
In this context, new technologie such as AI, IoT and blockchain are getting attention from professionals who are looking for the most efficient solutions to meet the goals. The benefits of blockchain in healthcare have been researched and it’s time to look at ways to practically implement the technology for the pharma supply chain to help solve the traceability issue.
First, let’s take a look at the regulatory landscape that shapes the face of the pharma supply chain.
Regulatory requirements to crack the traceability nut
In the US, for example, the DSCSA (Drug Supply Chain Security Act) requires all partners in the supply chain to exchange all transaction information electronically, securely and in an interoperable way. The transaction data is to include TI (Transaction Information), TH (Transaction History) and TS (Transaction Statement). Additionally, products receive a unique identifier. The regulations also call for a verification system and are to be implemented by November 2023.

In the EU, the FMD (Falsified Medicines Directive) includes similar requirements. Here, medical devices have also been impacted by the move towards stricter regulations. What used to be recommendations for best practices are now regulations enforceable by law. The MDR (Medical Device Regulations) also call for the introduction of traceability and identification systems and databases and increased post-market product surveillance.
In other words, it all circles back to traceability. If you can trace medication back all the way to the original supplier of raw materials, the chances of ending up with a counterfeit product are reduced – provided you can trust the data.
Why relying on regulations isn’t enough
Serialization and track & trace measures make it harder for bad industry players. However, it does not protect consumers from counterfeit medications and it does not ensure data integrity. As long as data remains alterable, the problem isn’t solved.
Another issue to keep in mind is that regulations are not synchronized. While they all have similar goals, each country or region has their own set of specific rules and manages data in different systems. In the US, the data is provided by the manufacturer and each player in the chain adds on top of it. In the EU, data is fed into a centralized database, the European Medicines Verification System (EMVS).
Authenticity and integrity of data remain an issue.
How can blockchain be used in the pharma supply chain?
One way of ensuring data remains untampered throughout the entire pharma supply chain is blockchain technology. Data entered into the distributed ledger is immutable. No one can change, but authorized partners in the chain can verify transaction history and add transactions. And that’s what the information flow in a supply chain is all about: a chain of transactions.
“A public blockchain is a shared database of all past transactions,” says Andreas Schindler, blockchain expert at Merck Group. “Plenty of participants have a copy of this database, so no one can just revert a transaction or make a change in the database as the rest of the community would say, ‘Your version of the blockchain is not correct, it doesn’t match with ours’.”
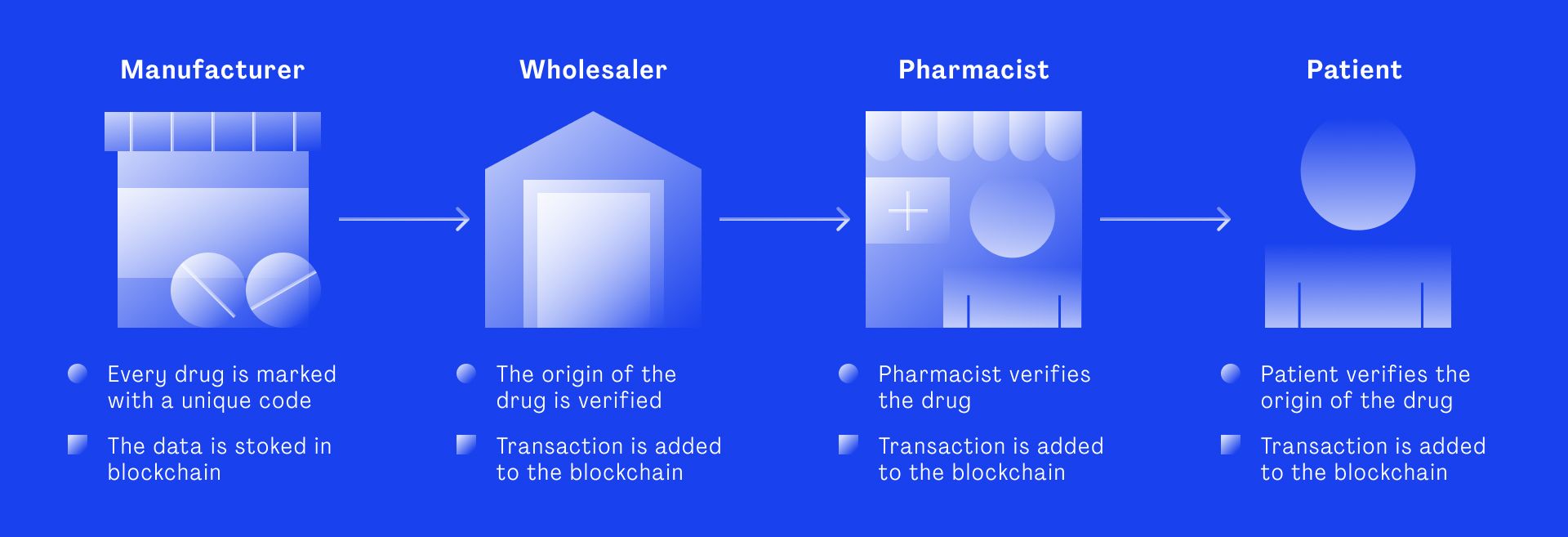
But what makes transaction data in the blockchain safe? Let’s say the shipper receives a delivery of several boxes of medicines and enters the transaction data into the system. The data includes the serial number, the origin, destination, shipper identity and validation data etc. Before this transaction gets accepted into the blockchain, the data gets verified and approved by the network. Does it match the previous transaction record – the previous block on the chain? Does the shipper meet the required transport conditions for this medication? Is the shipper authorized to transport this medication?
The computers in the network verify the data and if it matches, the transaction goes through and gets stored in a new block that is time-stamped and sealed through encryption. This means each block receives a hash, which is a unique multi-digit number. Each new block also includes the hash of the previous block. If anyone tried to enter a transaction that is not based on the data in the previous block, the hash would be different and therefore rejected by the network.
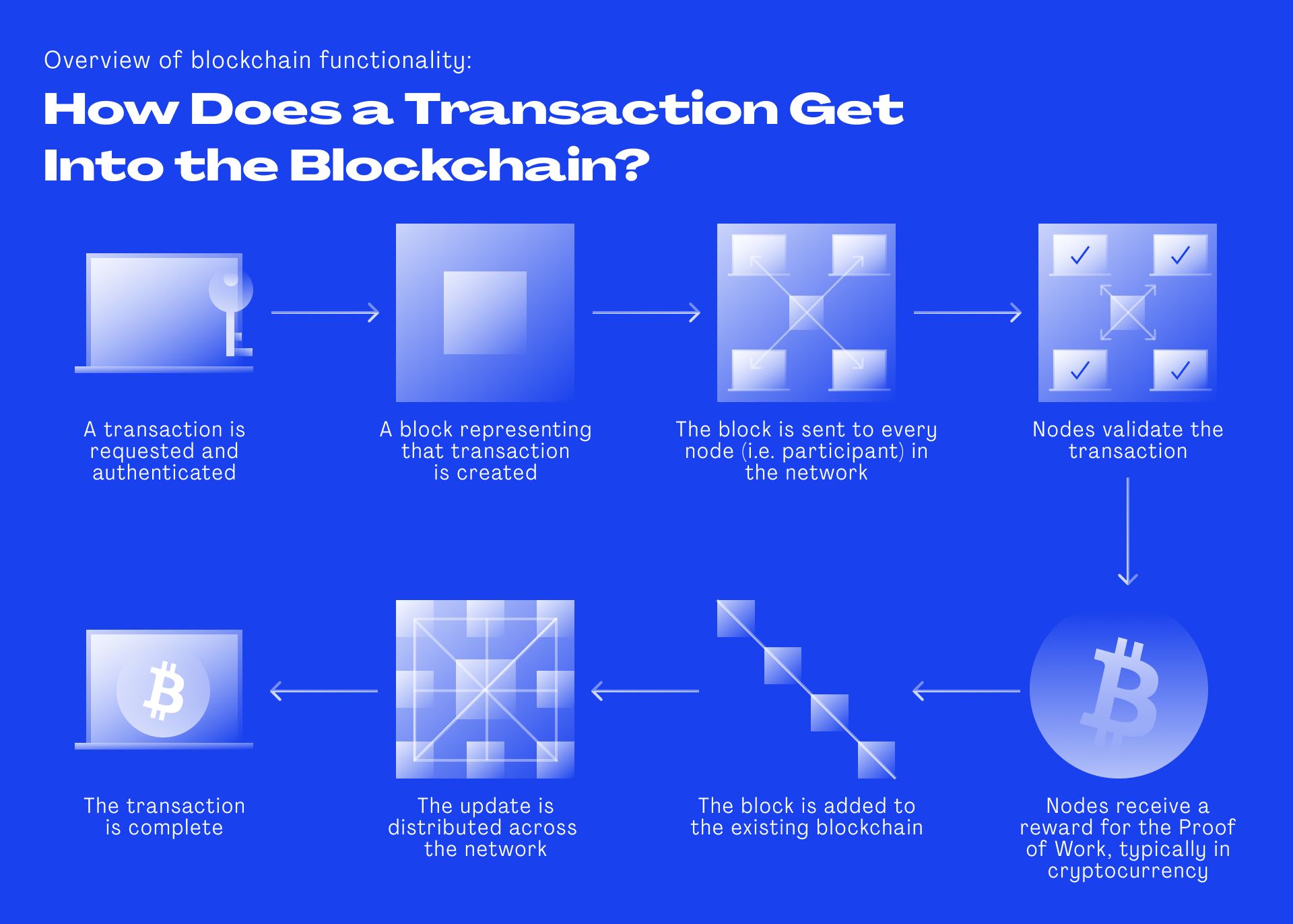
“The interesting thing is that you can always trace back any transaction in the blockchain to its origin,” explains Schindler. “Let’s say I had a token and I hand it over to someone and then they hand it over to the next person and so on, everybody along this chain can always trace back the token to me. And this makes it very relevant when thinking about how the supply chain works.” says Shindler.
This token can represent a specific package of a specific medication or an ingredient in that specific medication.
Refocus from crypto to blockchain pharmacy
“I think the biggest challenge to creating something like this [a blockchain] in the pharmaceutical supply chain is the mindset shift needed,” says Schindler. “Researchers, academia, drug manufacturers and all the distributors in their supply chain networks would need to work together. And that’s a big change from how we operate today.”
So, the challenge for blockchain in the pharma industry – or any other sincere industry – isn’t only on the technological level but also the way we think about blockchain. We need to refocus from cryptocurrency towards the blockchain’s ability to create an environment of trustworthy data and reliable transactions.
In the long run, blockchain technology will not only make the pharmaceutical industry safer for consumers, it can also bring about a dramatic reduction in insurance fees, contract costs, and other expenses.
Recent research on blockchain use in pharmacy
A recent academic study conducted at the University of Abu Dhabi, UAE proposes a framework that uses blockchain in supply chain management along with the Internet of Things (IoT). The method includes sensor technology to ensure appropriate conditions during transportation and storage. Through the execution of smart contracts the predefined contractual terms can be automatically verified at any time.
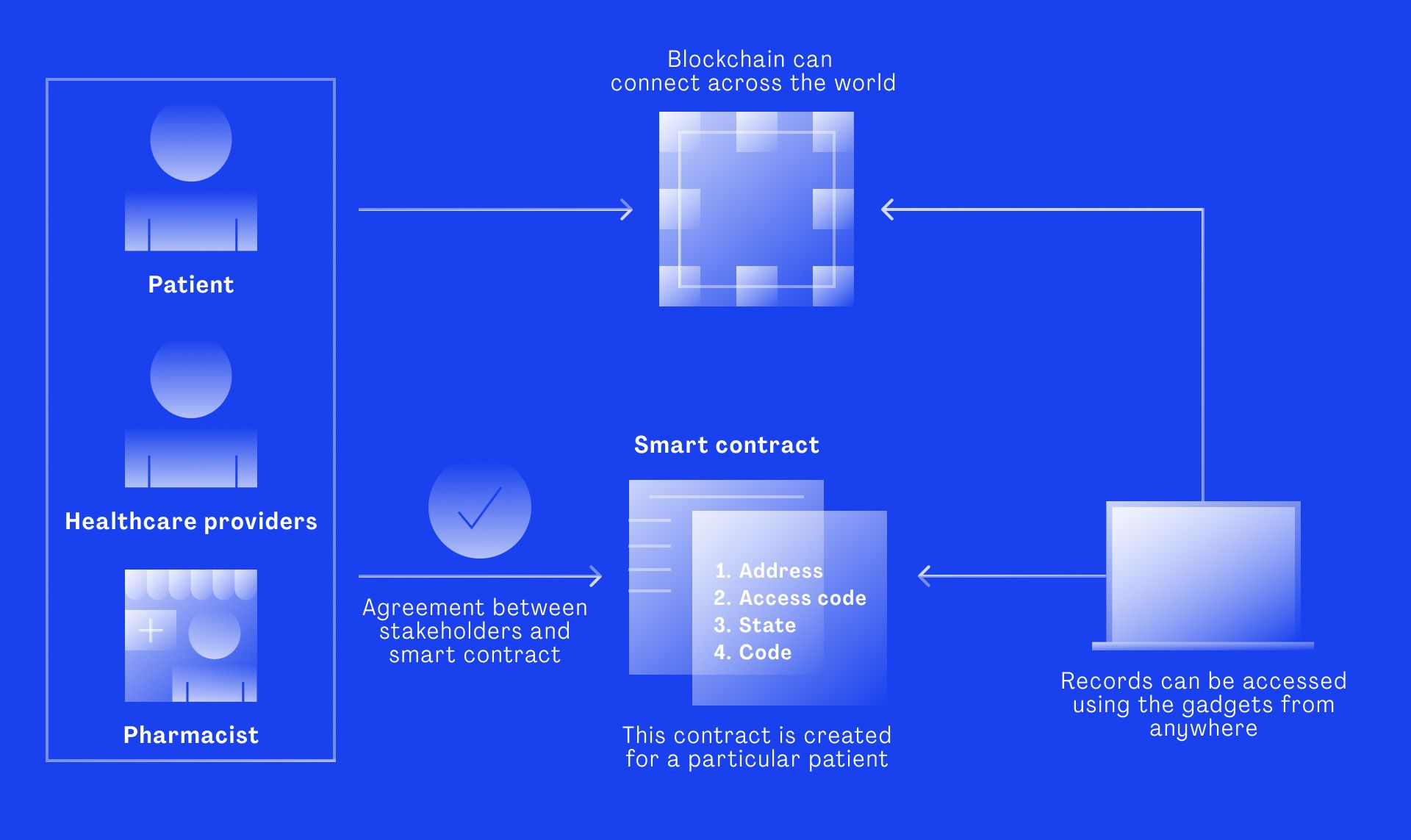
The solution eliminates the need for a third-party authenticator and ensures that all conditions are met. As such it is beneficial for all involved parties from raw material suppliers all the way to the patient.
Another research paper, also published early this year, investigated the use of a blockchain application prototype by a large pharmaceutical manufacturer in Egypt. The company employs 8,000 people and exports medicines including tablets, syrups, hard gelatin capsules, ampoules, sachets, creams and other sterile products to over 50 countries across the globe.
The study found that using the blockchain app had a positive impact on transaction costs. Contracting costs, processing costs. Also, lead times were reduced and the safe delivery of medications ensured. The technology also affects the governance of the supply chain by enhancing collaboration between involved parties and important stakeholders.
5 Examples of blockchain in pharmacy supply chain
There are several blockchain pharmacy apps in use already and more are being developed.
- The MediLedger Network is a global consortium of leaders in the pharmaceutical sector and counts Pfizer, Gilead, Genentech, Amgen and others among its members. The network has completed a pilot scheme of blockchain pharmacy supply chain management, demonstrating how the technology can improve the tracking of ownership changes in real-time and with confidentiality.
- Infosys Pharma Supply Chain Distributed Application is designed to provide a seamless network for suppliers, manufacturers, regulators, QA lab and logistics operators on a shared ledger to establish provenance for specialty drugs at package level and reduce revenue leakage to pharmaceuticals.
- Sonoco is a leading global provider of packaging for pharmaceutical distribution including temperature assuring solutions associated with IBM Pharmaceutical. The company is building a blockchain-based network.
- Another logistics supplier, CEVALogistics has partnered with Maersk and IBM to provide blockchain-enabled solutions for the shipping of pharmaceuticals.
- OCEASOFT, a company which designs atmospheric monitors, and Chronicled, an American blockchain firm, formed a collaboration. Their solution monitors the environmental conditions across the pharmaceutical supply chain leveraging blockchain and sensor technology.
Next steps in DeSci
You’ve taken a full spin through the DeSci landscape. From business models to supply chain, you are now well-equipped to take a deeper look into the industry. Research is in our blood, and so we designed a granular report so detailed that it will position you to launch a business or invest with confidence in the sector. What are you waiting for? Hit that arrow below and let’s go!